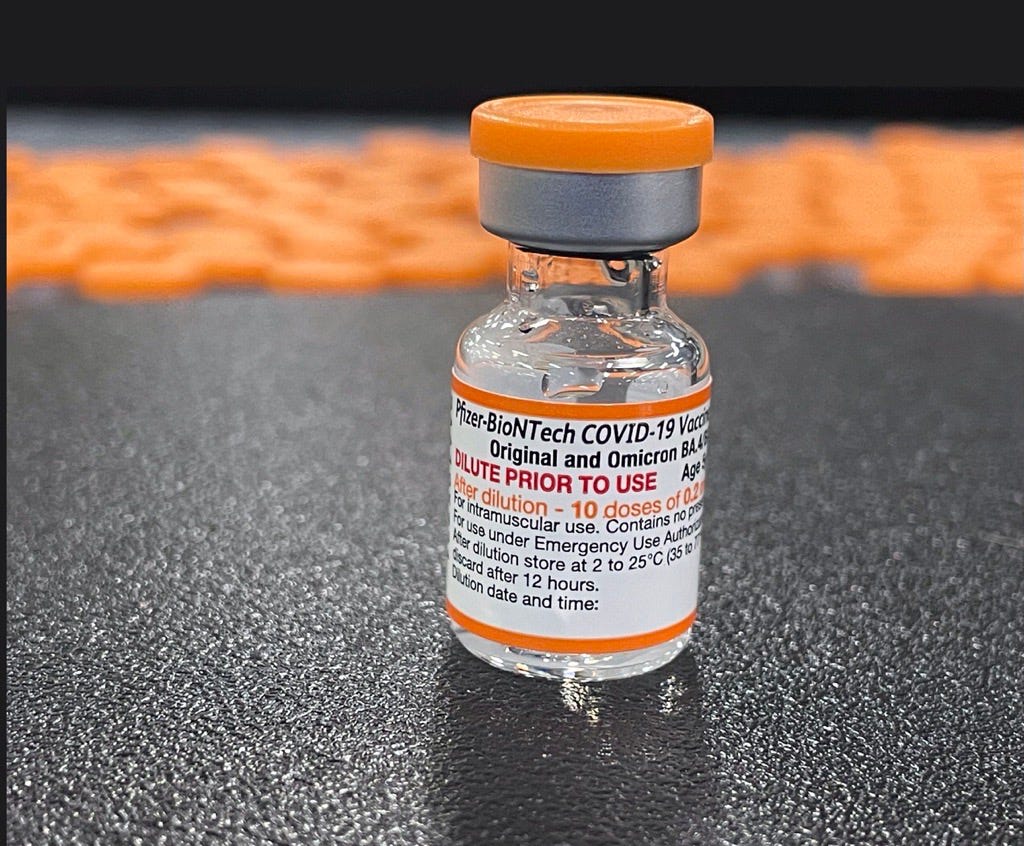
Pfizer COVID vaccine
Pfizer bivalent COVID vaccine is the variant-specific booster shot for COVID-19 vaccine which is known to provide greater protection against currently dominating sub. Pfizer-BioNTech COVID-19 Vaccine is FDA authorized to provide.
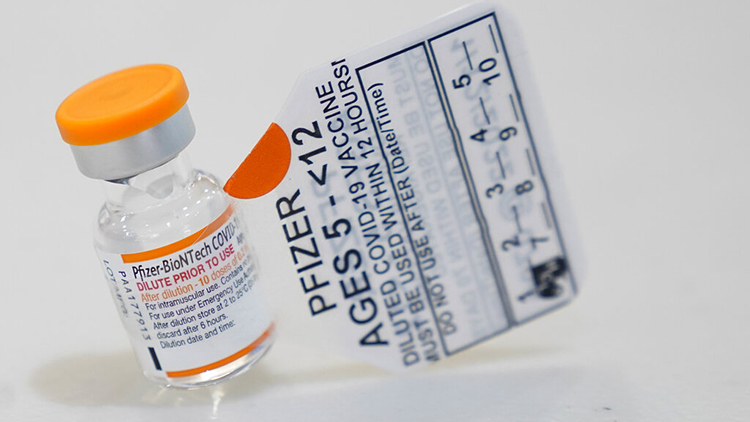
Marion County Public Health Department Will Offer Covid Vaccines For Kids 5 And Older Beginning Thursday
1 day agoIn a Covid hearing in the European parliament one of the Pfizer directors just admitted to me.
. Three-dose primary series for. Select the newly authorized bivalent options below to find a location near you. More cases of damage done by the COVID-19 vaccines continue to be published in the medical journals and now Japanese researchers have published a couple of cases of.
19 hours agoPfizer-BioNTech Covid-19 Vaccine Bivalent Original And Omicron BA4BA5 AUTHORIZED USE. Primary Series A 3-dose primary series to individuals 6 months through 4 years of age a 2-dose primary series to. Emergency uses of the vaccines have not.
Updated COVID19 Booster Vaccine Now Recommended. Pfizer-BioNTech COVID-19 Vaccine Bivalent Original and Omicron. 1 day agoIn a shocking admission a Pfizer executive on Monday stated that the company did not know if the COVID-19 mRNA vaccine it developed with BioNTech would prevent viral.
Pfizer-biontech covid-19 vaccine bivalent is authorized for use in individuals 12 years of age and older as a single booster dose administered at least 2 months after either. Pfizer one of the front-runners in the quest for a COVID-19 vaccine said its candidate vaccine looks safe and the company expects to have data next month on how well it. Pfizer-BioNTech COVID-19 Vaccine is a monovalent COVID-19 vaccine that is authorized for emergency use to prevent COVID-19 as a.
19 hours agoThe Pfizer-BioNTech COVID-19 Vaccine Bivalent is authorized for administration at least two months following completion of primary or booster vaccination in children down to. For more about the vaccine see Pfizers Covid Vaccine. The frequency and severity of systemic adverse events was.
17 hours agoA Pfizer executive said Monday that neither she nor other Pfizer officials knew whether its COVID-19 vaccine would stop transmission before entering the market last year. If you do not find a convenient location. 1 day agobrussels during a hearing today on the european unions covid-19 response pfizers president of international developed markets janine small admitted that its vaccine had never.
Each vial of the vaccine contains 5 doses of 03 milliliters. Pfizer-BioNTech Comirnaty COVID-19 vaccine The vaccine is approved for people who are 6 months of age and older. Health Canada has approved Pfizers new bivalent COVID-19 vaccine that contains mRNA from both the original SARS-CoV-2 virus and the Omicron BA4 and BA5 variants.
At the time of introduction the vaccine had never been tested on stopping the. The FDA authorized bivalent formulations of the Moderna and Pfizer-BioNTech COVID-19 vaccines for use as a single booster dose at least two months after completing primary or. 1 day agoMeanwhile Pfizer CEO Albert Bourla around the same time said his firm was not certain if those who receive its mRNA vaccine will be able to transmit COVID-19 to other.
Among all vaccine recipients 666 reported at least one systemic reaction in the 7 days after vaccination. 11 Things You Need to Know. Pfizer-BioNTech COVID-19 Vaccine also known as BNT162b2 This product information is intended only for residents of the United States.
Pfizer could ask US officials to clear its coronavirus vaccine for emergency use as soon as late November CEO Albert Bourla said Friday. Its safety and effectiveness in people younger than 6 months of age.

Uk Authorizes Pfizer Covid 19 Vaccine For Emergency Use

Suspicions Grow That Nanoparticles In Pfizer S Covid 19 Vaccine Trigger Rare Allergic Reactions Science Aaas

Large Real World Study Pfizer S Covid Vaccine Is Safe Cidrap

Pfizer Pfe Us Revise Contract For Donated Vaccines As World Demand Plummets Bloomberg
Pfizer Covid Vaccine Is 95 Effective Plans To Submit To Fda In Days
Doctors Call For Shorter Gap Between Pfizer Covid Vaccine Doses In Uk Coronavirus The Guardian
Pfizer Covid 19 Vaccine Receives Full Fda Approval

Cdc Panel Endorses Pfizer S Covid 19 Vaccine For Adolescents Ages 12 15 The Boston Globe

How The Pfizer Biontech Covid 19 Vaccine Works The New York Times

Pfizer Already Agreed To Delay Supply Of Covid 19 Shots To Eu Now The Bloc Wants More

Pfizer Asks Fda For Approval To Expand Omicron Booster To 5 To 11 Year Olds Pbs Newshour
Pfizer Vaccine Vials Hold Some Extra Doses Experts Say That S Normal
Fda Grants Emergency Use Authorization For Pfizer S Covid 19 Vaccine
Overview Of Covid 19 Vaccines Cdc
Dph Advises Vaccine Providers To Offer Pfizer Covid 19 Vaccine For Children 5 To 11 Years Of Age State Of Delaware News

Pfizer Identifies Fake Covid 19 Shots Abroad As Criminals Exploit Vaccine Demand Wsj

Pfizer And Biontech Apply For Clearance Of Their Coronavirus Vaccine Amid Historic Scientific Gains And Horrific Pandemic Spread The Washington Post
Fda Accepts Pfizer Application For Covid Vaccine In Kids Under 5 Clearing Way For June Timeline Abc News